A mechanism causing anergy of CD8 and CD4 T lymphocytes
The identification of specific tumor antigens recognized by T lymphocytes on human cancer cells has elicited numerous clinical trials involving vaccination of tumor-bearing cancer patients with defined tumor antigens. These treatments have shown a low clinical efficacy. Among metastatic melanoma patients, about 5% show a complete or partial clinical response following vaccination, whereas an additional 10% show some evidence of tumor regression without clear clinical benefit. We believe that progress depends on unraveling the different blockages for efficient tumor destruction.
The tumors of the patients about to receive the vaccine, already contain T cells directed against tumor antigens. Presumably these T cells are exhausted and this impaired function is maintained by immunosuppressive factors present in the tumor. The T cell response observed in some vaccinated patients reinforce an hypothesis proposed by Thierry Boon and Pierre Coulie: anti-vaccine CTL are not the effectors that kill the tumor cells but their arrival at the tumor site containing exhausted anti-tumor CTL, generates conditions allowing the reawakening of the exhausted CTL and/or activation of new anti-tumor CTL clones, some of them contributing directly to tumor destruction. Accordingly, the difference between the responding and the non-responding vaccinated patients is not the intensity of their direct T cell response to the vaccine but the intensity of the immunosuppression inside the tumor. It is therefore important to know which immunosuppressive mechanisms operate in human tumors.
Human tumor-infiltrating T lymphocytes
show impaired IFN-γ secretion
Both human CD8 and CD4 tumor-infiltrating T lymphocytes (TIL)
were isolated from tumor ascites or solid tumors and compared
with T lymphocytes from blood donors. TIL secrete low levels
of INF-γ and other cytokines upon non-specific stimulation
with anti-CD3 and anti-CD28 antibodies. TCR were observed to
be distant from the co-receptors on the cell surface of TIL,
either CD8 or CD4, whereas TCR and the co-receptors
co-localized on blood T lymphocytes.

TCR and CD8 do not co-localize on CD8 T cells with impaired functions
Reversing the anergy of
tumor-infiltrating T lymphocytes with galectin ligands
We have attributed the decreased IFN-γ secretion to a reduced
mobility of T cell receptors upon trapping in a lattice of
glycoproteins clustered by extracellular galectin-3. Indeed,
we have shown that treatment of TIL with N-acetyllactosamine
(LacNAc), a galectin ligand, restored this
secretion.
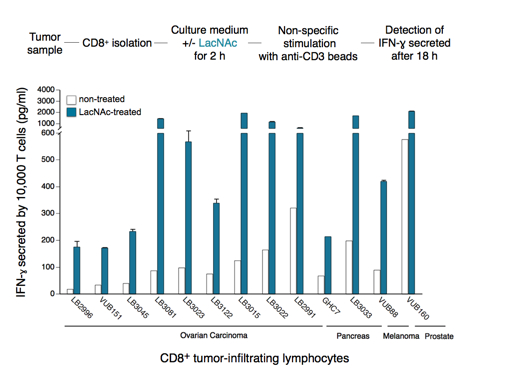
Treatment of tumor-infitrating lymphocytes with a galectin-competitor ligand reverses anergy
Our working hypothesis is that TIL have been stimulated by antigen chronically, and that the resulting activation of T cells could modify the expression of enzymes of the N-glycosylation pathway, as shown for murine T cells. The chronically activated TIL, compared to resting T cells, could thus express surface glycoproteins decorated with a set of glycans that are either more numerous or better ligands for galectin-3. Galectin-3 is an abundant lectin in many solid tumors and carcinomatous ascites, and can thus bind to surface glycoproteins of TIL and form lattices that would thereby reduce TCR mobility. This could explain the impaired function of TIL. The release of galectin-3 by soluble competitor ligands would restore TCR mobility and boost IFN-γ secretion by TIL. We recently strengthened this hypothesis by showing that both CD4 and CD8 TIL that were treated with an anti-galectin-3 antibody, which could disorganize lattice formation, had an increased IFN-γ secretion compared to untreated cells.
Towards a clinical trial combining
vaccination and galectin-binding polysaccharides
Galectin competitor ligands, e.g. disaccharides LacNAc, are
rapidly eliminated in urine, preventing their use in vivo. We
recently found that a plant-derived polysaccharide, currently
in clinical development, detached galectin-3 from TIL and
boosted their IFN-γ secretion. Importantly, we observed that
not only CD8+ TIL but also CD4+ TIL that were treated with
this polysaccharide secreted more IFN-γ upon ex vivo
re-stimulation. In tumor-bearing mice vaccinated with a tumor
antigen, injections of this polysaccharide led to tumor
rejection in half of the mice, whereas all control mice died.
In non-vaccinated mice, the polysaccharide had no effect by
itself. These results suggest that a combination of
galectin-3 ligands and therapeutic vaccination may induce
more tumor regressions in cancer patients than vaccination
alone. Translation of these results to the clinic was
unfortunately impossible because the company producing this
polysaccharide got bankrupted. We recently identified another
plant-derived polysaccharide that binds to galectins and was
already used in combination with chemotherapy in phase II
clinical trials in colorectal cancer patients. This compound
was as effective as LacNAc in boosting the secretion of IFN-γ
by treated TIL. A clinical trial with this new compound, in
combination with anti-tumoral vaccination, will hopefully be
launched in 2011 in clinical centers close to the Brussels
Branch. We are currently trying to understand the very early
activation events that are defective in TIL.
Further reading
Demotte N, Wieërs G, Van Der Smissen P, Moser M, Schmidt CW, Thielemans K, Squifflet J-L, Weynand B, Carrasco J, Lurquin C, Courtoy PJ, van der Bruggen P. 2010. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Research, 70: 7476-7488
Demotte N., Stroobant V., Courtoy P.J., Van der Smissen P., Colau D., Luescher I.F., Hivroz C., Nicaise J., Squifflet J.-L., Mourad M., Godelaine D., Boon T. and van der Bruggen P. 2008. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity, 28: 414-424
Demotte N, Colau D, Ottaviani S, Godelaine D, Van Pel A, Boon T, van der Bruggen P. 2002. A reversible functional defect of CD8+ T lymphocytes involving loss of tetramer labeling. European Journal of Immunology, 32: 1688-1697
< back - = - next >
